PREEMPT CRC Study
The largest clinical validation study for a blood-based CRC screening test


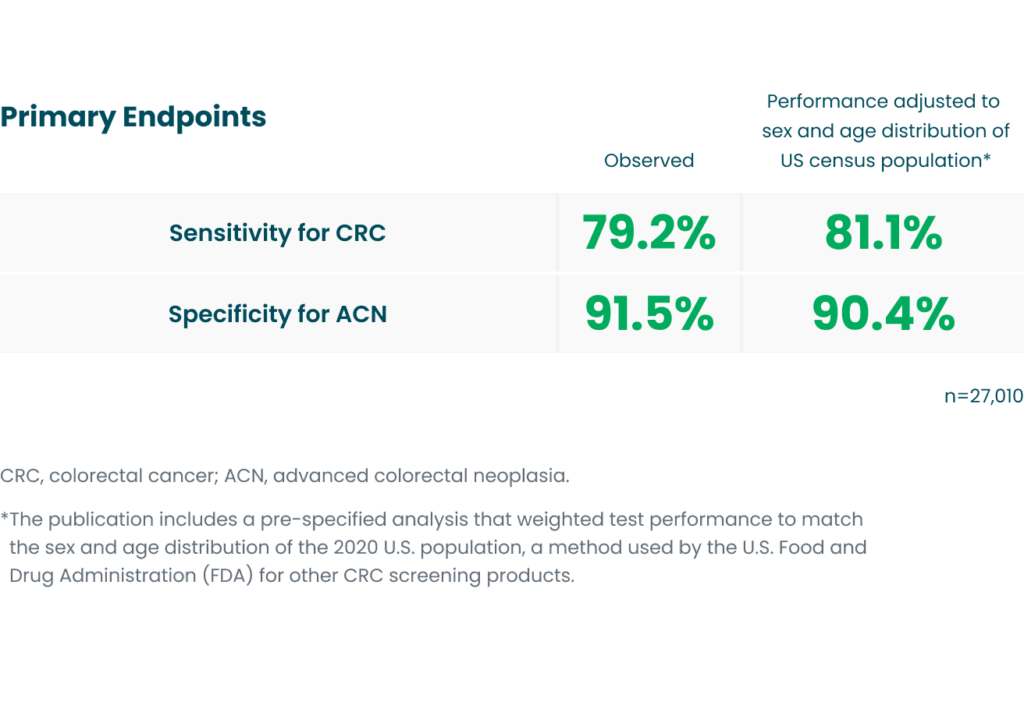
Study results
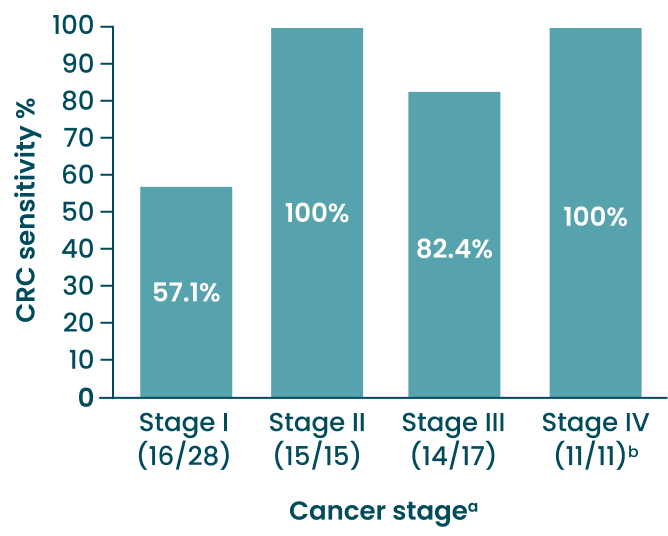
Test Sensitivity by CRC Stage
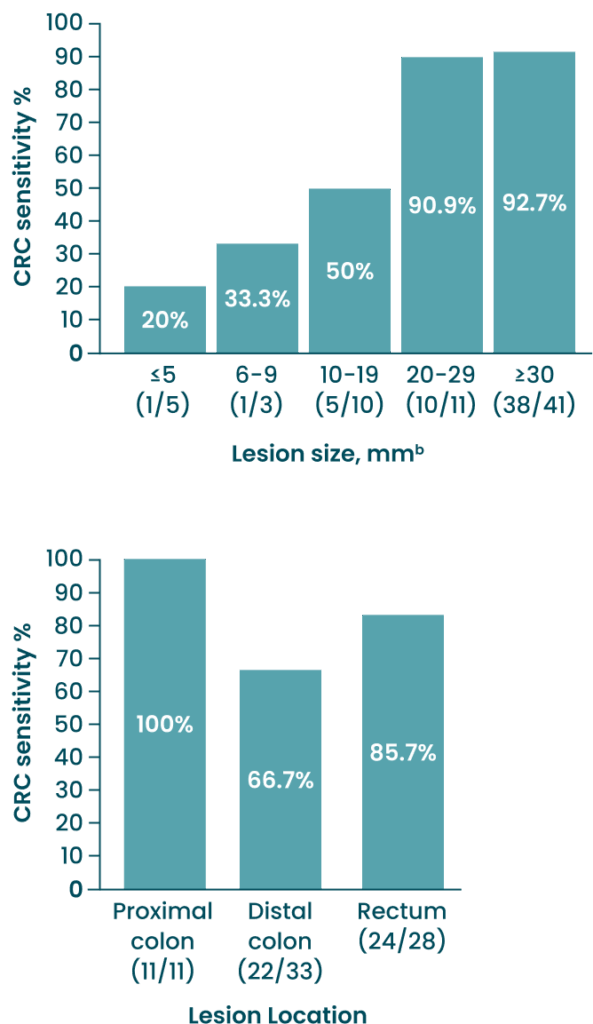
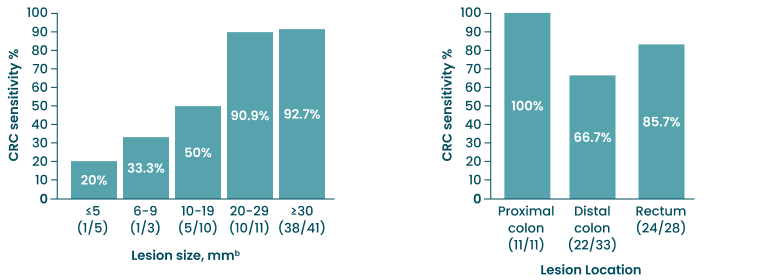
Test Sensitivity for CRC by Lesion Size and Location
Study recruitment
LARGE
REPRESENTATIVE
DIVERSE
>40kparticipants (of average risk for CRC)
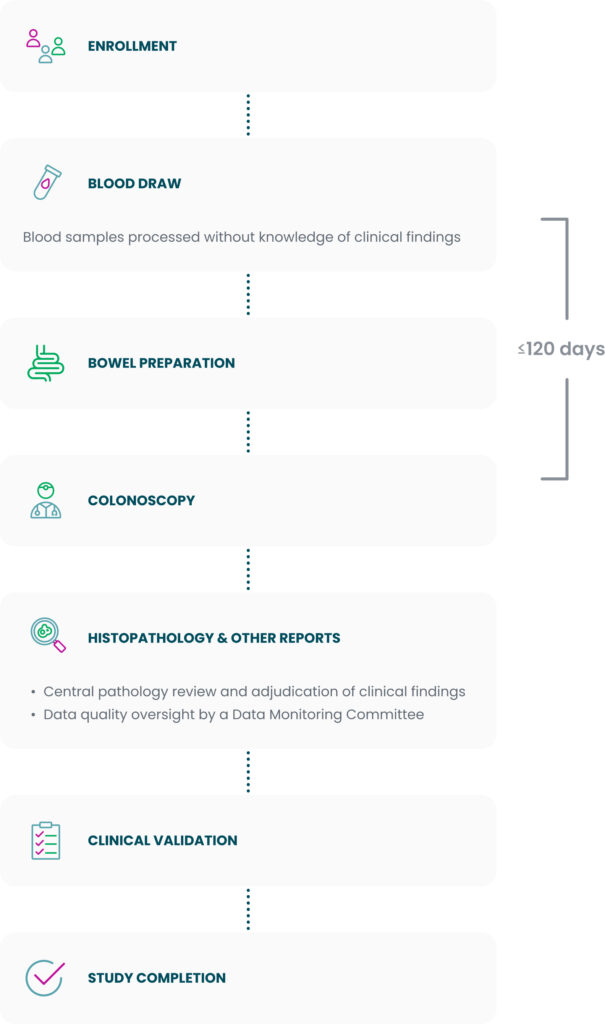
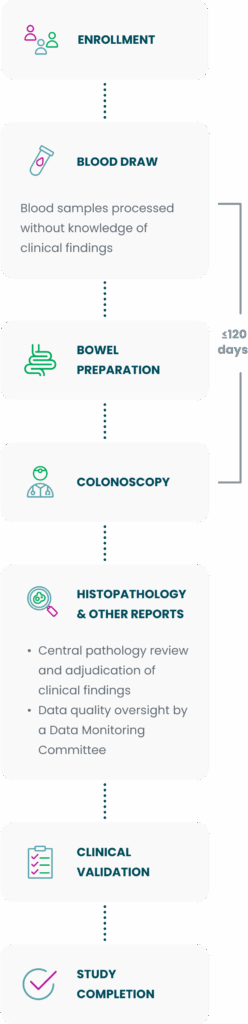
Study design
In the PREEMPT CRC study, blood draw results were clinically validated against colonoscopy-confirmed outcomes
Study population
Adults 45-85 years of age with average risk for CRC
- No personal history of cancer, colorectal adenoma, or inflammatory bowel disease
- No hereditary gastrointestinal cancer syndrome
- No high-risk family history of CRC
- Screen-eligible
How our screening platform works
Freenome developed a multiomics platform that analyzes genomic, epigenomic, and proteomic biomarkers to detect cancer-specific signals in the bloodstream, including tumor-derived cell-free DNA (cfDNA).
The CRC screening blood test was built on this platform and applies an AI/ML-based model to detect specific methylation signatures in cfDNA at single base resolution.
Why it matters
aStage was reported for all except one CRC case, which was deteced by the blood test. Stages were defined by the American Joint Committee on Cancer Staging System, 8th edition. CRC, colorectal cancer.
bLesion size was reported for all except two CRC cases.
References
1. Shaukat A, Burke CA, Chan AT, et al. Clinical validation of a circulating tumor DNA–based blood test to screen for colorectal cancer. JAMA. 2025;334(1):56-63. doi:10.1001/jama.2025.7515
2. Siegel RL, Krat§er TB, Giauinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322caac.21b71
3. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233-254. doi:10.3322caac.21772